Chemical Compound
-
Home
-
Chemical Compound
Chemical Compound
Visualizing the Hidden Architecture of Molecules: A Journey Through Higher Dimensions
The Molecular Realm Beyond Our Senses
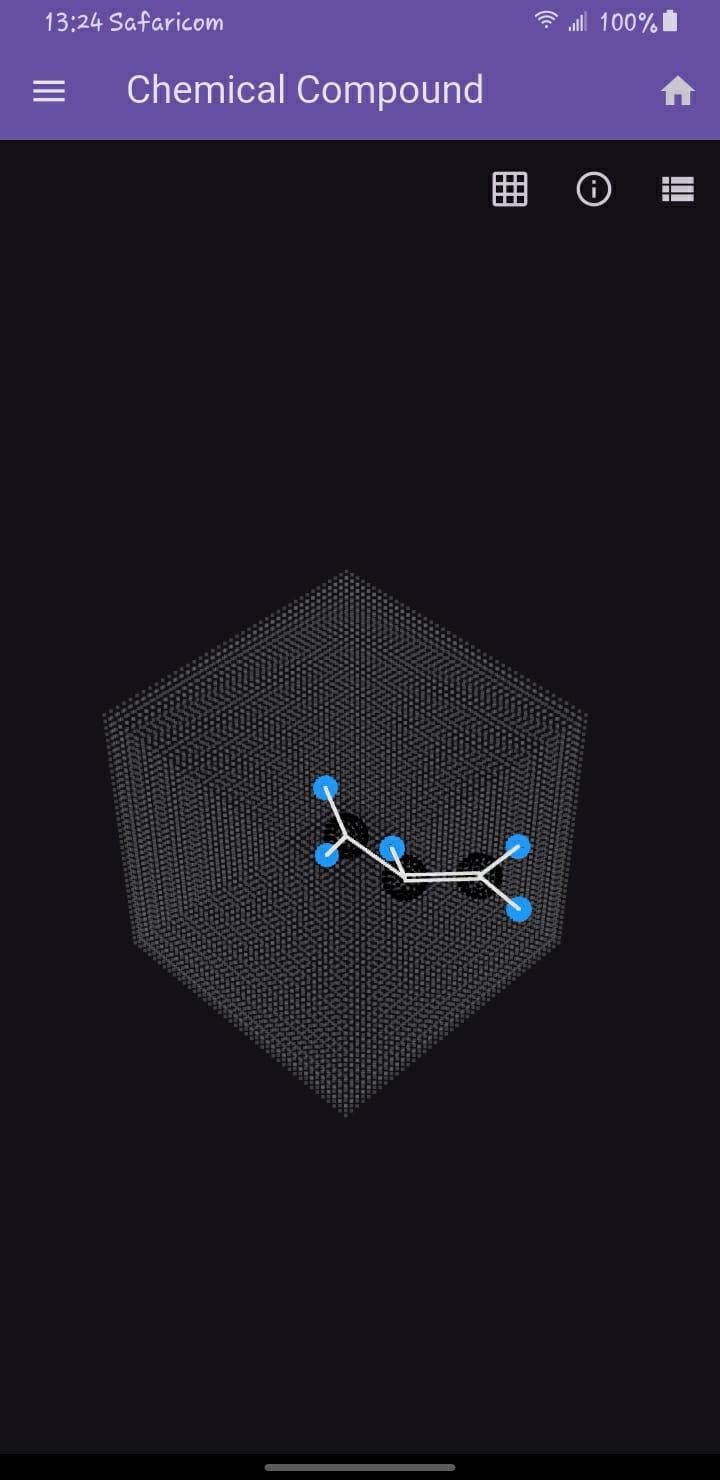
When we observe a simple molecule like propene (C₃H₆), we're glimpsing a reality that operates on fundamentally different principles than our macroscopic world. At the atomic scale, the familiar rules of geometry give way to quantum mechanical behaviors where electrons exist as probability clouds and chemical bonds form through shared electron densities. This molecular landscape exists in a higher-dimensional space that we can only comprehend through sophisticated visualization techniques.
The Fourth Dimension: Time in the Molecular World
In nature, molecules are never static. They vibrate, rotate, and undergo constant quantum fluctuations. A complete understanding requires adding time as a fourth dimension to our mental model. Through animation, we can observe:
Continuous rotation revealing the full three-dimensional structure
The dynamic nature of electron orbitals that constantly shift and reform
Bond vibrations that occur at femtosecond timescales in reality
This temporal dimension transforms our view from a static snapshot to a living system, much closer to how molecules actually behave.
The Fifth Dimension: Probability and Electron Clouds
The spherical distributions around each atom represent something profound - the higher-dimensional probability space of electrons. Unlike planets orbiting a sun, electrons don't follow fixed paths but rather exist as:
Three-dimensional standing waves described by quantum numbers
Probability densities where we can only predict likelihoods of position
Orbital shapes (s, p, d) that define chemical behavior
These fuzzy, probabilistic regions are mathematical constructs in a space our brains didn't evolve to visualize directly.
The Sixth Dimension: Bonding and Molecular Interactions
Chemical bonds themselves represent a higher-dimensional concept. The visualization distinguishes between:
Sigma (σ) bonds: Cylindrical electron densities along the internuclear axis
Pi (π) bonds: Sideways overlapping orbitals in double/triple bonds
Steric interactions: The spatial constraints that determine molecular shape
This dimensional understanding explains why double bonds resist rotation and how molecular geometry emerges from these quantum interactions.
The Seventh Dimension: Conformational Space
Every molecule can adopt multiple arrangements called conformations. For propene, this includes:
Different rotational states around single bonds
Transition states during chemical reactions
Interactions with neighboring molecules
This vast "conformational space" represents all possible arrangements the molecule can adopt, a landscape that chemists navigate when designing new materials or drugs.
The Scaffold of Reality
The coordinate system surrounding the molecule serves as more than just a reference frame - it represents:
The laboratory environment where measurements occur
Crystallographic axes in solid materials
The finite boundaries of molecular orbitals
This scaffold grounds our abstract quantum concepts in measurable reality.
Why This Matters
Understanding molecules through this multidimensional lens allows us to:
Design new materials with tailored properties
Develop life-saving pharmaceuticals
Create sustainable energy solutions
Decipher the molecular machinery of life
As we push the boundaries of nanotechnology and quantum computing, this ability to think in higher molecular dimensions becomes increasingly vital. The simple propene molecule contains within it the conceptual tools we need to navigate the coming revolutions in materials science, medicine, and technology.
By training our minds to see through these dimensional layers, we gain access to nature's most fundamental building blocks - not as static balls and sticks, but as dynamic, multidimensional quantum systems whose full complexity we're only beginning to appreciate.
Share this service: